Light Heating
Heat with light bulbs and measure temperature with a laser beam
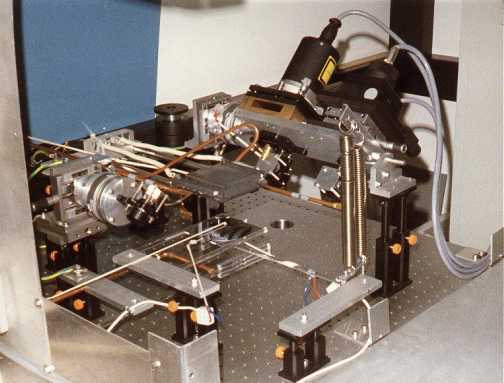
Measuring device, instrumental set-up
Entirely touch-free temperature measurement with an
ellipsometer. Reference dimension was the change of polarization of
a laser beam caused by change in temperature. Twice it run through
a 1 mm thin quartz pane, which could be heated by halogen lamps.
Its refraction angle of the inner total reflection changed with
temperature. The 180° turn of the beam after the first run through
the glass pane and the entry to the detector after the second run
on the way back were done by prisms.
A calibration curve with polarization change over temperature and respective
time was the goal.
Measuring temperature with an ellipsometer
The power of light - almost like in a tanning salon
Measurement set-up in working condition
Extraordinary effort! - Why? Thermocouples need a good
contact to the measuring object surface. Bad then, if the surface pressure
is limited. Together with a low heat conduction capability of the
measuring object or its surface (e.g. due to coating) the
thermocouple moreover acts like a cooling fin or under fast
temperature changes like a thermal load. The deviation can easily
reach values beyond ±10 °C (± 18 °F) or in the
two-digit percent range.
And pyrometers? They measure the heat radiation of an object and
calculate the temperature out of the Stefan-Boltzmann Law, see
below, and the area of the measurement spot. This radiation property,
however, strongly depends of the object material and is incorporated by the
correcting factor emissivity 0 < ε ≤ 1. Shiny metal
surfaces provide an ε much lower than 0.1, the black body
(approximately e.g. carbon black) a value of 1. Usually at least
0.7 is preset. Besides one should not underestimate the distortion
caused by external irradiation (e.g. reflections and other sources
in the field of view analog to a video camera) and standing waves
in transparent layers.
The light bulb as heating element
Electrical light bulb - the better stove: seven 500 W
halogen bulbs easily achieve 1 200 °C
(2 192 °F) within seconds. In industry and especially in semiconductor
processing this setup is often used for rapid thermal
processing or annealing (RTP, RTA).
The main connection between irradiation and heat is given by
Planck's law of radiation. In wave-length form (wave-length λ in m) it
provides for the temperature depending spectral irradiation energy
density of a black body (the object with the highest possible
emissivity)
r(λ, T) dλ = 2 π h c2/λ5 (exp (h c / (k T) λ-1) -1)-1 dλ
with
Planck constant h = 6.6262 10-34 Js
speed of light c = 2.99792 108 m
Boltzmann constant k = 1.3807 10-23 J/K
absolute temperature T in Kelvin
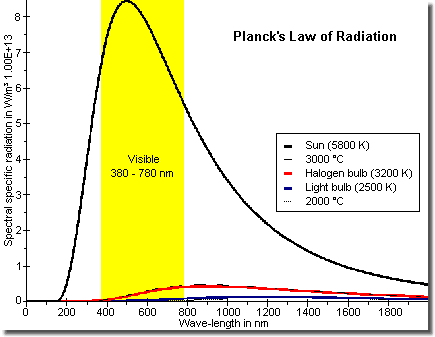
Efficiency of sun and light bulbs in comparison
Displayed in the figure is the irradiation of a black body after
Planck with the temperature of the sun surface (about
6 100 °C, 11 012 °F), that of a
filament of a standard light bulb (about 2 800±200 °C,
1 472±392 °F) and that of a halogen bulb (about
3 500±300 °C, 6 332±572 °F).
The visible light region is highlighted in yellow.
Integrated over all wave-lengths one gets with the Stefan-Boltzmann law the temperature depending irradiance
R(T) = ∫ r(λ, T) dλ = σ T4
with
Stefan-Boltzmann constant σ = 5.670 10-8
W/(m2 K4)
absolute temperature T in Kelvin
(Putting the area A [m2] of the black body as
multiplier into the formula above yields the radiation power.)
When (numerically) integrating over the visible light wave-length region 380 - 780 nm of the light bulb (temperature of the filament 2 500 K) one calculates a efficiency of about 6%. With the halogen bulb (temperature of the filament 3 200 K) one receives an efficiency of about 16%. Taking in account the low sensitivity of the human eye at the etches of the visible region, here especially the infrared, the »literature values« of about 3% for a 100 Watt bulb and maximal 8 to 10% for halogen bulbs, resp., seem to be comprehensive.
Fluorescent tubes, energy saving tubes and LEDs
Emitting of the gas light media is not done by a
heated source, but by electron pumping, see Franck-Hertz experiment.
In the in principle comparable fluorescent tubes and energy saving
tubes free electrons accelerated by the mains voltage hit electrons
circling around their nucleus and with sufficient energy kicking
them on a higher orbital. After the relaxation time of
10-8 seconds these electrons return to their former
orbital by emitting an ultraviolet photon with energy hf. The
opaque special coating of the glass tube converts these UV
irradiation to visible white light. These lamps show a more or less
continuous spectrum, thus covering all wave-lengths.
The semiconductor material of light emitting diodes (luminescence
diodes, LEDs) is directly electrically pumped. Recombination emitted
photons with the energy hf are closely arranged around a frequency
or a wave-length, resp., about some ten nanometers, thus they
are monochromatic.
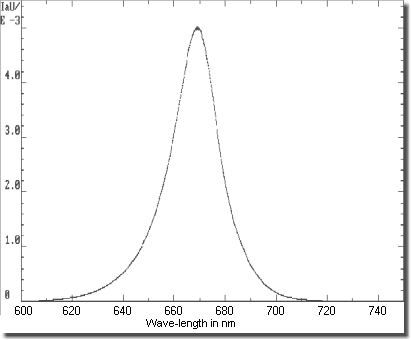
Measured spectrum of a red super-bright LED
The spectrum of the 640 nm GaAlAs
super-bright Kingbright L-53SRC-E LED measured with a (grating)
monochromator in arbitrary units shows good accordance with the
data sheet values - i.a. the maximum is found at about
660 nm. (Maybe due to the not well calibrated measurement
setup ;-)
The completely depicted section lies in the monochrome red. That
is the efficiency improvement compared to bulbs (incl. halogen)
with their very wide spread spectrum shown above.
By the way: in this plot the spectrum of a laser LED would
degenerate to a single small (but very high ;-) impulse spike
instead of the bell-shaped curve.
For white light one has to bundle a red, a green and a blue LED,
either in separate housings or integrated in one and the same.
(Often there are two blue LEDs due to their comparatively low
efficiency.) A discontinuous spectrum is the result.
There is a similar possibility compared to fluorescent tubes.
Often a efficient blue LED illuminates a special yellowish coating,
which then together emit white light with a halfway continuous
spectrum. The often
circulated »tails« into the UV section then obviously are
possible.
The technical development does not pause. More and more
powerful, intelligent connected and well designed LED lamps are
available. And for cars there are laser headlights already.
